G4G Resources
ACE's Guidelines for Guidelines (G4G) resources are intended to equip guideline developers with knowledge to meet the nine minimum standards to create evidence-based, high-quality guidelines.
G4G self-appraisal checklist
Refer regularly to the G4G self-appraisal checklist, which details how to attain G4G standards and serves as the basis for ACE's methodological validation.
G4G toolkit
Whether you're new to guideline development or looking to strengthen your approach, this curated G4G toolkit summarises relevant resources and materials aligned with internationally recognised quality criteria.
To get an overview of G4G standards and their meaning, read the G4G guide and watch the webinar “Building Trust through Rigour: Essential Standards for Developing High-Quality Clinical Guidelines”.
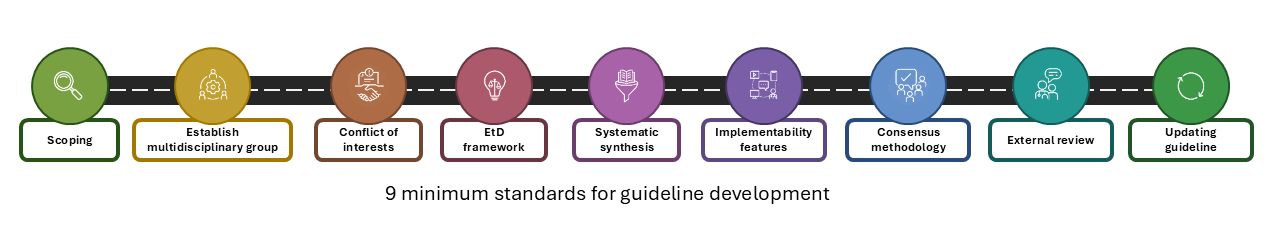
Click below on individual G4G areas and explore the recommended resources.
Scoping
Select a relevant and useful scope
Developing NICE guidelines: the manual – The scope is a useful and comprehensive resource to better understand how to focus your guideline scope. For further reading, please refer to the resources below.
Additional reading materials:
Establish a multidisciplinary expert group
Establish an appropriate multidisciplinary group with clearly stated role(s) in the guideline development
The composition of the multidisciplinary group will depend on several factors, such as the guideline topic and scope, and may vary across guideline organisations. Below, we listed useful reading and resources to guide you when appointing your guideline panel:
Conflicts of interest
Collect and manage conflicts of interest
Each guideline organisation has its own conflict of interest (COI) policies and management approaches, reflecting different organisational priorities and governance structures. The Guideline International Network (GIN) has published core principles for disclosure and management of COI in guidelines in this useful paper: Guidelines International Network: Principles for Disclosure of Interests and Management of Conflicts in Guidelines | Annals of Internal Medicine.
Other references and examples of how reputable guideline bodies approach COI declaration and management are listed below:
GIN McMaster Checklist – Conflict of Interest Considerations
NICE – Declaring and managing interests: advisory committees
WHO Handbook for guideline development – “Declaration and management of interests”
Evidence to Recommendation (EtR) framework
Use evidence to guide recommendations, underpinned by a pre-determined evidence-to-decision framework
The “GRADE Handbook – Going from evidence to recommendations” is one of the most comprehensive and commonly used resource guiding how to formulate recommendations from evidence and serves as an excellent starting point.
Other useful readings are listed below, including approaches to the evidence-to-decision/recommendation (EtD/R) step used by reputable guideline bodies:
GIN McMaster Checklist – Summarising Evidence and Developing Recommendations
Developing NICE guidelines: the manual – Interpreting the evidence and writing the guideline
WHO Handbook for guideline development – “Evidence assessment” and “Developing recommendations”
If you are new to GRADE, the GRADEpro Platform can help supporting the EtR process and is helpful in standardising your approach.
ACE regularly publishes EtRs alongside our guidelines; here’s an example from the osteoporosis ACG. Our EtR approach has been presented during the 2025 AVBC conference, you can watch the video here.
Systematic synthesis
Use systematic literature review methods for synthesising evidence
The “Cochrane Handbook for Systematic Reviews of Interventions” offers comprehensive and professional guidance to systematically synthesise evidence and serves as an essential reference for this standard .
Below, other useful readings are listed, including evidence synthesis methods used by reputable guideline bodies:
GIN McMaster Checklist – Deciding what Evidence to Include and Searching for Evidence
WHO Handbook for guideline development – “Evidence retrieval and synthesis”
Several tools exist to assess risk of bias. Here are some commonly used ones:
For systematic reviews and meta-analyses: CASP Critical Appraisal Checklists
For randomised controlled trials: Cochrane Risk of Bias (RoB 2) tool
For observational studies: ROBINS-I V2 tool
For observational studies: Newcastle-Ottawa Scale
Implementability features
Include implementability features
Successful guidelines go beyond providing evidence-based recommendations—they are designed to be easily implemented in practice. While approaches vary across organisations, the resources below describe how to integrate implementability features throughout the development process, from planning to publication.
Consensus methodology
Use a consensus methodology
Each guideline organisation employs its own approach to achieve consensus. Below, we listed useful readings to better understand the various methods, including the consensus approaches applied by reputable guideline bodies:
Developing NICE guidelines: the manual - Making group decisions and reaching consensus
WHO Handbook for guideline development – “Decision-making in the guideline development group”
If you decide to apply the RAND/UCLA consensus methodology, ACE has developed an in-house template for collating ratings and automatically calculate agreement – click here .
External review
Seek external review before publication
Various stakeholders can be engaged for external review processes, depending on the guideline scope, audience and organisational requirements. The resources below provide guidance on how to effectively seek and manage external feedback before publication, including approaches used by reputable guideline bodies:
WHO Handbook for guideline development – “The external review group” and “Peer review”
Example: WHO Guideline on Low Back Pain – Contributors to the Guideline
Updating guidelines
Plan for updating the guideline
The resources below provide guidance on key considerations for determining when and how to update your guideline.
A note from ACE – methods for developing the G4G toolkit: a comprehensive search for relevant published articles and methodological documents across academic databases and grey literature sources was conducted. Focused searches were performed in PubMed, Cochrane Library, Google, and Google Scholar using targeted keyword combinations without date restrictions. The searches were conducted in December 2025. Only articles published in English were included in the search.
Two authors independently conducted the searches and screened for relevant methodological articles covering different stages of guideline development. Additional targeted searches were performed across relevant professional guideline organisations and their associated websites. The G4G toolkit will be maintained as ‘live’ document, regularly updating the information with additional resources as needed.
G4G video resources
AMS 2025 Webinar Series on Practice Guidelines: Building Trust Through Rigour: Essential Standards for Developing High-Quality Clinical Guidelines [26 Nov 2025]
AVBC 2025 Pre-conference Workshop: Bridging the gap in developing trustworthy clinical guidelines: Linking evidence to recommendations [16 Oct 2025]
VBHC 2024 Pre-conference Workshop: Best Practices for Clinical Guidelines Development [22 Aug 2024]
AMS 2025 Webinar Series on Practice Guidelines: Building Trust Through Rigour: Essential Standards for Developing High-Quality Clinical Guidelines
In collaboration with AMS, Director (ETP) and Senior Lead Specialist (ETP) explore the importance of using standardised approaches to guideline development to ensure rigour and successful implementation of guidelines in Singapore. This video covers a broad overview of the nine G4G standards.
AVBC 2025 Pre-conference Workshop: Bridging the gap in developing trustworthy clinical guidelines: Linking evidence to recommendations
Join ACE’s Evidence to Practice division as they walk through the use of evidence to recommendation frameworks to structure the best available evidence and provide transparency in decision making. This video addresses G4G standard 4 – Use evidence to guide recommendations, underpinned by a pre-determined evidence-to-decision framework.
VBHC 2024 Pre-conference Workshop: Best Practices for Clinical Guidelines Development
Re-visit Professor Zachary Munn's workshop on best practices for clinical guidelines development, which took place at the Value-Based Healthcare Conference 2024.
